



































TLW's Chemical Elementscope™ (Chemical Element Historyscope) |
By T.L. Winslow (TLW), the Historyscoper™ |
© Copyright by T.L. Winslow. All Rights Reserved. |
Original Pub. Date: Mar. 31, 2016. Last Updated: Apr. 25, 2024. |


















Westerners are not only known as history ignoramuses, but double dumbass history ignoramuses when it comes to chemical element history. Since I'm the one-and-only Historyscoper (tm), let me quickly bring you up to speed before you dive into my Master Historyscope.
In the 1st cent. C.E. only nine chemical elements are known (copper, lead, gold, silver, iron, tin, sulfur, mercury, zinc), vs. 12 in 1500, 19 in 1750, 84 in 1900, and 100 in 1953 (year of TLW's birth) - maybe it's a govt. coverup? Today 118 elements are known, incl. #115 Ununpentium (2003), #116 Livermorium (2000), #117 Ununseptium (2010), and #118 (Oganesson) (2002).

In 1250 German Dominican friar-priest philosopher-scientist (St.) Albertus Magnus (Albert the Great of Cologne) (1193-1280) (teacher of Thomas Aquinas) discovers the element Arsenic (As) (Gr. "yellow orpiment") (#33), going on to "give Aristotle to the Latins" with tons of essays on every branch of science, philosophy, and theology, citing Maimonides and Avicenna.

In 1557 Italian scholar-physician Julius Caesar Scaliger (1484-1558) discovers the metallic element Platinum (pt) (#78)

In 1669 alchemist Hennig Brand (Brandt) (1630-1710) of Hamburg, Germany discovers Phosphorus (P) (#15) in distilled human urine, becoming the first new element discovered since ancient times, launching the chain of events leading to the Periodic Table - he wasn't kidding about his burning urination?

In 1730 Swedish chemist Georg Brandt (1694-1768) discovers Cobalt (Co) (#27), becoming the second new element discovered since ancient times (phosphorus in 1669).


In 1744 Spanish scientists slash naval officers Antonio de Ulloa (1716-95) and Jorge Juan y Santacilia (1713-73), who went on the La Condamine expedition to South Am. in 1736 then remained, discover sizeable stores of the element Platinum (Pt) (#78) (Sp. "platina" = little silver), and take some back to Europe with them on separate ships; too bad, Ulloa's ship is captured by the Brits, but after the scientists pull their increasingly powerful internat. strings he ends up a fellow of the Royal Society of London - what's it good for, doorstops?



In 1771 German-Swedish chemist Karl (Carl) Wilhelm Scheele (1742-86) discovers the halogen element Fluorine (Lat. "fluo" = to flow) (F) (#9); in 1774 he discovers Ammonia, Barium (Ba) (#56), Chlorine (Cl) (#17) and Baryta (barium sulfate), and, along with English chemist Joseph Priestley (1733-1804) and French chemist Antoine Lavoisier (1743-94) the element Oxygen (O) (#16), which is first isolated by Priestley on Aug. 4 by focusing sunlight through a lens onto mercuric oxide (red calx), calling it "dephlogisticated air", finding that breathing it gives him an uplifted sensation, with the soundbyte: "‘The feeling of it in my lungs was not sensibly different from that of common air, but I fancied that my breast felt peculiarly light and easy for some time afterwards"; the belief that there are just four elements (earth, fire, water, and air) is exploded with the discovery that air is a mixture of gases.
In 1778 Carl Wilhelm Scheele discovers the element Molybdenum (Mo) (#42), and proves that molybdenite (molybdenum sulfide), graphite (black lead or plumbago), and galena (lead II sulfide) are three different minerals.
In 1772 Scottish chemist Daniel Rutherford (1749-1819) discovers the gaseous element Nitrogen (N) (#14).

In 1774 Swedish chemist-metallurgist Johan Gottlieb Gahn (1745-1818) of Falun, Sweden discovers Manganese (It. "by metathesis") (Mn) (#25).


In 1781 Spanish (Basque) brother chemists Don Fausto Fermin de Elhuyar (Delhuyar) y De Lubice (1755-1833) and Juan Jose de Elhuyar (1754-96) discover the mineral element Tungsten (Swedish "heavy stone") (W) (#74) in wolframite and scheelite, and Karl Wilhelm Scheele determines its composition; because of the wolframite connection it receives the German symbol W and is also called wolfram; it has the highest melting point of any metal (3380 C, 6116 F).

In 1782 the sulfur-like element Tellurium (#52) (Te) (Lat. "tellus" = earth) is discovered combined with gold in Transylvania by Austrian mineralogist Franz=Joseph Muller (Müller) von Reichenstein (1740-1826).

In 1789 German chemist Martin Heinrich Klaproth (1743-1817) makes the first chemical analysis of Pitchblende, and discovers the element Uranium (U) (#92), which initially is used in ceramics and textiles; he also discovers the metallic element Zirconium (Zr) (#40) in the sands of the rivers of Sri Lanka (Srilankium?); uranium is later used to make fuel elements for atomic reactors, and zirconium in containers for fuel elements since it doesn't absorb neutrons.

In 1791 English Anglican minister and mineralogist William Gregor (1761-1817) of Cornwall, England discovers the metallic element Titanium (Ti) (#22) (melting point 3,272 F) in (ilmenite) (FeTiO3) from the Manaccan Valley, and names it manaccanite; in 1795 Martin Klaproth of Germany rediscovers it in rutile from Hungary, and names it for the Greek Titans.

In 1793 after it is discovered in 1790 by physician Adair Crawford (1748-95) and surgeon-chemist William Cruickshank (-1811) in the village of Strontian, Scotland, Scottish physician-chemist Thomas Charles Hope (1766-1844) names the highly reactive alkaline earth metallic element Strontium (Sr) (#38), calling it Strontites, after which on June 30, 1808 Sir Humphry Davy isolates it from strontium chloride and renames it.

In 1799 English scientist Sir Humphry Davy (1778-1829) proves that two pieces of ice rubbed together will melt without the addition of any heat, disproving the Caloric Theory of Heat; on Dec. 26 he becomes the first to describe the mental effects of Laughing Gas (nitrous oxide); "I existed in a world of new-connected and newly modified ideas." In 1808 he uses electrolysis to isolate chemical elements Barium (#56) (Ba) (as an amalgam only - the pure metal is not isolated until 1901) and Strontium (#38) (Sr). In 1811 he discovers colorless poisonous heavy gas Phosgene (carbonyl chloride).

In 1802 the silver-white hydrogen-absorbing metallic element Palladium (Pd) (#46) (which is later alloyed with gold to make white gold) is discovered by English chemist-physicist William Hyde Wollaston (1766-1828), followed in 1804 by the the metallic element Rhodium (Rh) (#45), useful in strengthening platinum.

In 1803 English chemist Smithson Tennant (1761-1815) discovers the rare (#61) metallic elements Osmium (Os) (#76) and Oridium (Ir) (#77) in platinum ores; the specific gravity of Ir (22.4) is exceeded only by Os (24), which is the most dense known substance; they are later used in the alloy osmiridium to strengthen platinum.


In 1808 French Sorbonne prof. of physics (1808-32) Joseph Louis Gay-Lussac (1778-1850) and Baron Louis-Jacques Thenard (Thénard) (1777-1857) isolate hard nonmetallic element Boron (#5) (B); Gay-Lussac measures the relative volume of gases involved in chemical reactions, and next year formulates Gay-Lussac's Law of Combining Volumes, that the ratios of the volumes of reacting gases are small whole numbers; it is independently discovered by Jacques Charles - also true for gay-lez ass gasses?

In 1811 after his father is put in debtors' prison in 1805, forcing him to manage his saltpeter business, French chemist Bernard (Barnard) Courtois (1777-1838) courteously discovers the metallic element Iodine (I) (#53) in seaweed ash.

In 1817 Swedish physician-chemist Jons (Jöns) Jacob Berzelius (1779-1848) discovers the chemical non-metal element Selenium (Se) (#34), named after the Moon after noting similarities with tellurim (named after the Earth) without knowing that its electrical conductivity varies with the intensity of sunlight (makes glass red too).


In 1828 German chemist Friedrich Wohler (Wöhler) (1800-82) isolates the pure light gray metal element Beryllium (#4) (Be) (discovered in 1798); he also synthesizes the organic chemical urea from the inorganic chemical ammonium cyanate, founding organic chemistry and dealing a death blow to the vitalistic theory that there is a vital force in living materials which creates an impassible gulf with inorganic ones; too bad, the vitalists get a reprieve when they find that he cheated and got his raw material from bones, and it takes until 1850 to kill vitalism completely; French chemist Antoine Alexandre Brutus Bussy (1794-1882) independently isolates beryllium.

In 1829 German chemist Johann Wolfgang Dobereiner (Döbereiner) (1780-1849) pub. the existence of a simple relationship among the atomic weights of elements having similar properties - he's got the columns but not the rows?


In 1830 Swedish chemist Nils Gabriel Sefström (1787-1845) discovers the element Vanadium (#23) (V); in 1801 Spanish-Mexican mineralogist Andres Manuel del Rio Fernandez (1764-1849) (Andrés Manuel del Río Fernández) discovered it and called it erythronium; in ? Friedrich Wohler proves that it is the same as vanadium.

In 1838 Swiss chemist Carl Gustav (Gustaf) Mosander (1797-1858) discovers the rare-earth metallic element Lanthanum (La) (#57) (Gr. for hidden), followed in 1843 by Terbium (Tb) (#65) (#54 of 54 in the rare earth group) and Erbium (Er) (#68) (#50 in the rare earth group) in Ytterby, Sweden.

In 1842 the rare earth element Yttrium (Y) (#39) (obtained from monazite sand, known for use in red phosphors for color TVs and garnet crystals) is discovered by Swedish chemist Carl Gustaf (Gustav) Mosander (1797-1858), who also discovers the rare metallic element didymium in cerite, but later finds that it's just a mixture of the elements neodymium and praseodymium. In 1843 Carl Gustav Mosander discovers the rare-earth metallic elements Terbium (Tb) (#65) (#54 of 54 in the rare earth group) and Erbium (Er) (#68) (#50 in the rare earth group) in Ytterby, Sweden.

In 1844 German chemist Karl Ernst Claus (1796-1864) discovers the greyish-white metallic element Ruthenium (Ru) (#44) in platinum ores.
In 1845 Friedrich Wohler extracts pure metallic element Aluminum (Al) (#13) from clay.

In 1863 German chemists Ferdinand Reich (1799-1882) and Hieonymus Theodor Richter (1824-98) discover the metallic element Indium (In) (#49) using spectroscopy - rich rectal spectroscopy?

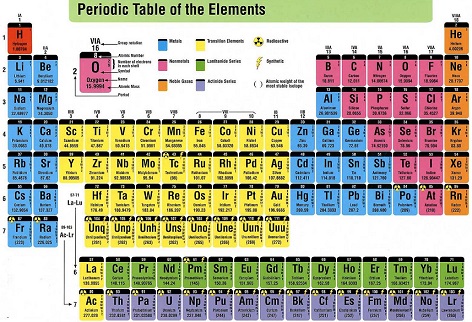
In 1863 English chemist John Alexander Reina Newlands (1837-98) (who just returned from fighting for Giuseppe Garibaldi in Italy) pub. the first Periodic Table of the Elements arranged in order of relative atomic masses, leaving open the possibly of undiscovered elements, and predicting the existence of germanium, scooping Dmitri Mendeleyev of Russia, although he is ridiculed.


In 1868 after an eclipse, French astronomer Pierre Jules Cesar (César) Janssen (1824-1907), along with English chemist Sir Edward Frankland (1825-99) and English astronomer Sir Joseph Norman Lockyer (1836-1920) of England discover a new element in the Sun's chromosphere which they call Helium (He) (#2); it takes until 1895 for Sir William Ramsay to prove that it exists on Earth.


In 1869 Russian chemist Dmitri Ivanovich Mendeleyev (Mendeleev) (1834-1907) pub. the Periodic Law and the Periodic Table of the Elements, which future chemistry students are face-forked with, boldly making room for the missing element gallium; its appearance is influenced by the new game of Solitaire? - so, village atheist, if everything happened by chance, and there is no intelligent designer, why is there a mathematical code behind matter itself? In 1871 Mendeleyev pub. The Periodic Regularities of the Chemical Elements, which boldly predicts that some accepted atomic weights are grossly in error, and other elements yet to be discovered will plug up gaps in his Periodic Table. In 1875 the first English account of his work is pub. in the London Chemical News in Dec.; his 1871 paper is first trans. and pub. in this journal in 1879-80. Which chemical element is named for a cock? In 1875 using zinc blende from the Pyrenees, French chemist Paul-Emile (Francois) Lecoq de Boisbaudran (1838-1912) discovers the metallic element Gallium (#31) (Ga), which resembles aluminum but can be cut with a knife (melting point 30.15 deg C), right where Mendelyeev had predicted an element with these properties would be found in a gap in the Periodic Table; he suggests the name "eka aluminum" for it, but the name gallium is chosen as a pun on the Latin word gallus (cock).


In 1878 Swiss chemists Marc Delafotaine (1837-1911) and Jacques-Louis Soret (1827-90) discover rare-earth element Holmium (Ho) (#67) (43rd most abundant element in the Earth's crust) in the spectrum of gadolinite; in 1879 Swedish chemist Per Teodor Cleve (1840-1905) chemically separates it from thulium and erbium - so rare it would take a Holmes to find it?


In 1878 Swiss chemist Jean Charles Galissard de Marignac (1817-94) isolates soft silver metallic rare earth element Ytterbium (Yb) (#70) from pure erbia, and names it after Ytterby, the Swedish town where he found it; in 1886 he discovers rare-earth (#40) chemical element Gadolinium (#64) (Gd), and names it after Finnish chemist Johan (John) Gadolin (1760-1852), who isolated gadolinite, the ore in which it is found near Falun, Sweden, Hitra Island, Norway, et al.

In 1879 Swedish chemist Lars Fredrik Nilson (1840-99) discovers metallic element Scandium (#21) (Sc) in wolframite eight years after Dmitri Mendeleyev predicted its existence based on the periodic law and predicted its properties as similar to boron.

In 1885 the rare earth metal element Neodymium (#60) (Nd) (which gives off pink light) is discovered by Karl Auer von Welsbach (1858-1929) of Germany.

On Feb. 6, 1886 the hard, brittle, reflective grey chemical element Germanium (#32) (Ge) is discovered by German chemist Clemens Alexander Winkler (1838-1904) in argyrodite after he figures out that the main components silver and sulfur only take up 93% of the mass, and initially calls it ekasilicon because it is chemically related to silicon; it ends up being named after Germany.

On June 26, 1886 French chemist Henri Moissan (1852-1907) isolates pure pale yellow Fluorine (F) (#9) (Lat. "fluo" = to flow) gas, earning him a prize of 10K francs from the French Academy of Science. In 1892 he invents the electric arc furnace, and uses its 3.5K C temp to produce tiny artificial diamonds along with new carbide, silicide, and boride compounds, winning the 1906 Nobel Chem. Prize.

In 1886 French chemist Paul-Emile (Francois) Lecoq de Boisbaudran (1838-1912) discovers the metallic rare earth element Dysprosium (Dy) (#66), known for the highest magnetic susceptibility of the rare earths.



In 1895 English Argonauts, er, scientists John William Strutt, 3rd Baron Rayleigh (1842-1919) and Sir William Ramsay (1852-1916) discover the first "noble" (inert) gas, chemical element Argon (Ar) (#18), isolated from air; Ramsay proves that helium (discovered in the Sun's atmosphere in 1868) exists on Earth in the uranium ore elevite; later it is found that it exists in all radioactive minerals as a result of radioactive decay, which emits alpha particles (helium nuclei). In 1898 the rare gas elements Krypton (Kr) (#36) (1 part in 20M in the atmosphere), Neon (Gr. "new") (Ne) (#10) (1 part in 65K in the atmosphere), and Xenon (Xe) (#54) are discovered by British chemists Sir William Ramsay and Morris William Travers (1872-1961) in liquid air.

In 1896 French chemist Eugene-Anatole Demarcay (1852-1903) discovers the hard silvery metallic element Europium (Eu) (#63) in samples of samarium, taking until 1901 to isolate it; meanwhile in 1898 he uses spectroscopy to confirm that Marie Curie has discovered the element radium.


In 1898 Polish-born French brain babe Marie Curie (1867-1934) coins the term "radioactivity"; she and her hubby Pierre Curie (1859-1906) discover the radioactive elements Radium (Ra) (#88) (named after radioactivity) (Dec. 21) and Polonium (Po) (#84) (named after her native Poland) in pitchblende from the St. Joachimsthal mines in Bohemia, winning them the 1903 Nobel Physics Prize; early medical uses found for radioactivity in cancer treatment cause it to be billed as a fountain of youth until its cancer-causing power is discovered; too bad, she dies on July 4, 1934 of leukemia caused by overexposure to radiation, and her lab has to be torn down brick by brick and her papers banned for half a cent. as too dangerous to handle without signing a medical release.

In 1898 German physicist Friedrich Ernst Dorn (1848-1916) of Halle discovers radioactive noble gas Radon (Rn) (#86) as a gas given off by radium, becoming the 3rd radioactive element discovered after radium and polonium.

In 1899 French chemist Andre Louis Debierne (1874-1949) discovers the radioactive element Actinium (#89) (Ac) in pitchblende, in which it occurs in the proportion of 1 part per 5B; it is discovered independently in 1902 by Friedrich Oskar Giesel (1852-1927) in Germany, which he calls emanium.

In 1906 Am. radiochemist Bertram Borden Boltwood (1870-1927) discovers the element Ionium, and shows it to be chemically identical to thorium, proving the existence of isotopes; in 1907 he uses the decay of uranium to lead to date rocks to ages between 400M and 2.2B years, becoming the first successful use of Radiometric Dating; the Nat. Academy of Sciences officially adopts it in 1926.


In 1906 the rare earth metal element Lutetium (Lu) (#71) is discovered by French chemist Georges Urbain (1872-1938) after he separates ytterbia (discovered in 1878 by Jean de Marignac) into neoytterbia (ytterbium) and lutecia (lutetium); Karl (Carl) Auer Freiherr von Welsbach (1858-1929) of Germany independently does the same thing, calling them aldebaranium and cassiopeium.




In 1913 the dense silver-gray radioactive metallic element Protactinium (Pa) (#91) (protoactinium until 1949) (naturally occurring in pitchblende) is discovered at the lab of Ernest Rutherford in Manchester, England by Polish chemist Kasimir (Kazimierz) Fajans (1887-1975) and German chemist Oswald Helmuth Goehring (Göhring) (1889-1915), who call it brevium because of its short half-life; in 1917 German physicists Otto Hahn (1879-1968) and Lise Meitner (1878-1968) discover a more stable isotope, and give it the name proto-actinium, which is changed to protoactinium in 1949 by IUPAC, meaning parent of actinium; it becomes a product of nuclear reactors, but has no known uses; in 1915 Scottish chemist John Arnold Cranston (1891-1972) discovered the most stable isotope, but delayed announcement after being called into military service for WWI.


In 1922 the rare (#47) metallic element Hafnium (Lat. "Copenhagen") (#72), naturally occuring in most zirconium minerals (5% strength) is discovered in Copenhagen by Dutch physicist Dirk Coster (1889-1950) and Hungarian chemist George Charles de (Georg Karl von) Hevesy (1885-1966) based on Niels Bohr's prediction that it should resemble zirconium in structure, causing them to look in guess what kind of ores.

In 1925 Walter Noddack (1893-1960) and Ida Noddack (1896-1978) of Germany discover the metallic element Rhenium (Rh) (#75); too bad, they also claim to isolate long-missing element #43, which they call masurium after Walter's family home in E Prussia, but their results can't be duplicated, and some get pissed-off at their nationalism, so others get credit in 1937 for technetium (Tc); in 1998 U.S. researchers confirm their results, so now what?
In 1931 the chemical element alabamine (#85) is claimed to be discovered by Alabama Polytechnic Inst. scientists; during WWII it is prepared synthetically by bombarding bismuth with alpha particles, and shown to be unstable and incapable of existing in Nature, and given the name Astatine (Gr. "unstable" + ine) (At).

In 1931 Am. physical chemist Harold Clayton Urey (1893-1981) discovers the hydrogen isotope Deuterium (Gk. "deuteros" = second) (heavy hydrogen, incl. a neutron in the nucleus), winning him the 1934 Nobel Chem. Prize.

In 1937 Italian mineralogist Carlo Perrier (1886-1948) and Italian physicist Emilio Gino Segre (Segrč) (1905-89) of the U. of Palermo in Sicily discover the metallic element Technetium (Tc) (#43) in discarded molybdenum foil from cyclotron parts of the Lawrence Radiation Lab, becoming the first artificially-produced element; the name panormium, after the Latin name for Palermo (Panormus) is chucked.



In 1940 Dale Rraymond Corson (1914-2012), Kenneth Ross MacKenzie (1912-2002), and Emilio Gino Segre (Segrč) (1905-89) of UCB synthesize the radioactive halogen element Astatine (At) (#85) (Gr. "astatos" = unstable).





In 1940 after Egon Bretscher (1901-73) and Norman Feather (1904-78) of the Cavendish Lab discover that a slow neutron reactor fueled with uranium can produce substantial amounts for use in an atomic bomb, the element Neptunium (Np) (#93) is first synthesized by Edwin Mattison McMillan (1907-91) and Philip Hauge Abelson (1913-2004) of the Berkeley Radiation Lab, becoming the first transuranic element synthesized; Neptune follows Uranus, therefore neptunium follows uranium? On Mar. 28, 1941 Glenn Theodore Seaborg (1912-99) and Edwin Mattison McMillan (1907-91) of the U.S. discover Plutonium (Pl) (#94), named after the planet not the dog; in 1944 Americium (Am) (#95) and Curium (Cm) (#96) are discovered by Seaborg et al. at the U. of Chicago and UCB.

In Dec. 1949 the radioactive chemical element Berkelium (Bk) (#97) is discovered by Stanley Gerald Thompson (1912-76) et al. at the U. of Calif. Radiation Lab by bombarding americium with high-energy alpha particles in a cyclotron; it is later prepared by neutron bombardment of plutonium.

Californicating with the basic elements again? On Mar. 17, 1950 U. of Calif. (Berkeley) scientist Glenn Theodore Seaborg (1912-99) announces the creation of the radioactive element Californium (#98) (Cf) by intense neutron bombardment of plutonium (curium?); the chemical element Berkelium (#97) (Bk) is prepared by bombarding americium with high-energy alpha particles in a cyclotron; it is later prepared by neutron bombardment of plutonium.

The Big 100 Year in Science? In 1952 the synthetic elements Einsteinium (Es) (#99) and Fermium (Fm) (#100) are discovered in the debris of the first H-bomb explosion by Albert Ghiroso (1915-2010) et al. of UCB and the Argonne Lab. In 1955 Ghiorso produces the element Mendelevium (Md) (#101) by bombarding einsteinium with high-energy alpha particles in a cyclotron. In 1958 Ghiorso produces the chemical element Nobelium (No) (#102) by the radioactive bombardment of curium in Stockholm. On Feb. 14, 1961 Ghiorso synthesizes the radioactive element (half-life 3.6 hours) Lawrencium (#103) (Lr).
In 1967 the George, er, Dubna Inst. in Russia creates the radioactive element Dubnium (Db) (#105) (longest-lived transactinide isotope - half life 28 hours) by shooting neon atoms at americium; on Apr. 27, 1970 U. of Calif. physicists synthesize it.
By 1968 a total of 103 elements have been discovered, which are adopted as a std.

In 1974 the Joint Inst. for Nuclear Research in Dubna, Russia and Lawrence Libermore Lab in Calif. co-discover chemical element #106, a homologue to tungsten, which is named Seaborgium (#106) (Sg) in honor of UCB scientist Glenn Theodore Seaborg (1912-99)





In 1976 Soviet scientists in Dubna create synthetic chemical element Bohrium (Bh) (#107) (original names eka-rhenium and unnilseptium) by hitting chromium atoms with bismuth atoms; too bad, nobody can verify their results, and in 1981 a team at the Inst. for Heavy Ion Research (Gesellschaft fur Schwerionenforschung Darmstadt) (GSI) in Darmstadt, Germany, led by Peter Armbruster (1931-) and Gottfried Muenzenberg (Münzenberg) (1940-) synthesizes it, becoming the ones who are officially recognized in 1992; since element #105 was given the name dubnium, the IUPAC goes with the German suggestion to honor Danish physicist Niels Bohr, but shorten the proposed name neislbohrium; Armbruster-Munzenberg go on to discover elements #108-#112. On Aug. 29, 1982 they announce the synthesis of radiactive synthetic element Meitnerium (#109) (Mt), named after Austrian physicist Lise Meitner (1878-1968). In 1984 they synthesize the synthetic radioactive element Hassium (Hs) (#108), named after the German state of Hesse; in 1994 they discover the radioactive synthetic elements Darmstadtium (Ds) (#110) and Roentgenium (Rg) (#111), named after German physicist Wilhelm Konrad Roentgen (Röntgen) (1845-1923); in 1998 they discover the radioactive synthetic element Copernicium (Cn) (#112), named after Polish astronomer Nicolaus Copernicus (1473-1543).
In 1998 the Int. Union of Pure and Applied Chemistry confirms six new elements added to the Periodic Table of the Elements: Rutherfordium (Rf) (#104) (1968), Dubnium (Db) (#105) (1970), Seaborgium (Sg) (#106) (1974), Bohrium (Bh) (#107) (1981), Hassium (Hs) (#108) (1984), Meitnerium (Mt) (#109) (1982) - rubadubdub in the boring sea tub, have some, mite?

In 1983 Lenoir, N.C.-born biochemist Kary Banks Mullis (1944-2019) discovers DNA Amplification, splitting the DNA in a single cell into two strands then starting a Polymerase Chain Reaction (PCR) that creates millions of identical pieces, becoming a breakthrough for genetic research, "virtually dividing biology into two epochs" (New York Times) and winning him a share of the 1993 Nobel Chem. Prize.

In 1998 scientists at the Flerov (Flyorov) Lab of Nuclear Reactions of the Joint Inst. for Nuclear Research in Dubna, Russia discover the superheavy artificial element Flerovium (Fl) (#114), named in honor of Russian (Soviet) physicist Georgy Nikolayevich Flyorov (1913-90).
In 2000 the superheavy synthetic radioactive element Livermorium (Lv) (#116) is discovered by the Lawrence Livermore Nat. Lab in Calif.; the name is adopted by IUPAC on May 30, 2012. In 2000 Leonid Khriachtchev (1959-), Markku Rasanen et al. of the U. of Helsinki in Finland report the first known stable compound of the inert noble gas argon, Argon Fluorohydride (HArF) by shining UV light on frozen argon containing a small amount of hydrogen fluoride, proving that it's not really so inert.
In 2000 Leonid Khriachtchev (1959-), Markku Rasanen et al. of the U. of Helsinki in Finland report the first known stable compound of the inert noble gas argon, Argon Fluorohydride (HArF) by shining UV light on frozen argon containing a small amount of hydrogen fluoride, proving that it's not really so inert.
On Oct. 9, 2002 scientists at the Joint Inst. for Nuclear Research (JINR) and Lawrence Livermore Nat. Lab announce the discovery of the new element (a halogen) Ununoctium (Uuo) (#118).

In 2002 the synthetic unstable radioactive chemical element Oganesson (Og) (#118) is discovered at the Joint Inst. for Nuclear Research (JINR) near Moscow by a joint team of Am. and Russian scientists, named after nuclear physicist Yuri Tsolakovich Oganessian (1933-).
In 2003 the Joint Inst. for Nuclear Research (JINR) in Dubna, Russia discovers the synthetic radioactive element Ununtrium (Uut) (#113) and Ununpentium (Uup) (#115).
On Oct. 9, 2006 after an initial report in 2002, scientists at Joint Inst. for Nuclear Research (JINR) in Dubna, Russia and Lawrence Livermore Nat. Lab in Calif. announce the discovery of the synthetic element (a halogen) Ununoctium (Uuo) (#118).
In 2010 the Joint Inst. for Nuclear Research (JINR) in Dubna, Russia discovers the synthetic radioactive element Ununseptium (Uus) (#117).
On May 30, 2012 chemical elements #114 Flerovium (Fl) (#114) (discovered in 1998 by the Flerov Lab of Nuclear Reactions in Dubna, Russia) and Livermorium (Lv) (#116) (discovered in 2000 by the Lawrence Livermore Lab in the U.S. and the Joint Inst. for Nuclear Research in Dubna, Russia) are officially named by the Internat. Union of Pure and Applied Chemistry (IUPAC).

On Mar. 21, 2016 P.R. Alexander et al. pub. an article in Nature that speculates that neutron star mergers may be the only way to form many elements heavier than zinc. On Nov. 30 the Internat. Union of Pure and Applied Chemistry (IUPAC) officially approves names for chemical elements Nihonium (Nh) (#113) (formerly Ununtrium), Moscovium (Mc) (#115) (formerly Eka-Bismuth), Tennessine (Ts) (#117) (formerly Eka-Astatine), and Oganesson (Og) (#118) (formely Eka-Radon), named after Russian nuclear physicist Yuri Tsolakovich Oganessian (1933-) (highest known atomic mass).