





































TLW's Chemistscope™ (Chemist Historyscope) |
By T.L. Winslow (TLW), the Historyscoper™ |
© Copyright by T.L. Winslow. All Rights Reserved. |
Original Pub. Date: Jan. 4, 2017. Last Update: May 21, 2024. |


















Westerners are not only known as history ignoramuses, but double dumbass history ignoramuses when it comes to chemistry history and chemist history. Since I'm the one-and-only Historyscoper (tm), let me quickly bring you up to speed before you dive into my Master Historyscope.
In this historyscope we will skip over most of the discovery of chemical elements and drugs and Nobel Prize awards and concentrate on discovery of chemical methods and compounds.
In the 1st cent. C.E. only nine chemical elements are known (copper, lead, gold, silver, iron, tin, sulfur, mercury, zinc), vs. 12 in 1500, 19 in 1750, 84 in 1900, and 100 in 1953 (year of TLW's birth) - maybe it's a govt. coverup? Today 118 elements are known, incl. #115 Ununpentium (2003), #116 Livermorium (2000), #117 Ununseptium (2010), and #118 (Oganesson) (2002).

About 1200 B.C.E. About this time Tapputi (Tapputi-Belatekallim) lives in Babylonian Mesopotamia, distilling and filtering perfumes and becoming the world's first known chemist.

In 782 Arab (al)chemist-physician ("Father of Arab Chemistry") Abu Musa Jabir (Geber) (Gebir) ibn Hayyan (721-815) begins to study chemistry as distinct from alchemy: calcination, oxidation, congelation, fixation, solution, digestion, distillation, evaporation, sublimation, separation, extraction, ceration, fermentation, putrefaction, propagation, projection. He dies after allegedly discovering methods for preparing sulfuric acid, nitric acid, aqua regia, and silver nitrate; by the 10th cent. 100+ anon. works are written using his name, after which Euro alchemists of the 12th and 13th cents. escape the Inquisition by doing ditto.

In 1250 German Dominican friar-priest philosopher-scientist (St.) Albertus Magnus (Albert the Great of Cologne) (1193-1280) (teacher of Thomas Aquinas) discovers the element Arsenic (As) (Gr. "yellow orpiment") (#33), going on to "give Aristotle to the Latins" with tons of essays on every branch of science, philosophy, and theology, citing Maimonides and Avicenna.
In 1361 French alchemist Nicolas Flamel (1330-1418) discovers the alchemical textbook The Sacred Book of Abraham the Jew, Prince, Priest, Levite, Astrologer and Philosopher to that Tribe of Jews Who by the Wrath of God Were Dispersed Amongst the Gauls, spending 21 years trying in vain to understand it until a converted Jew in Leon gives him the key; on Jan. 17, 1382 he performs his first successful chemical transmutation in Paris, growing wealthy, allowing him to endow 14 hospitals, seven churches, and three chapels in Paris, and ditto in Boulogne, making him a hit with Sir Isaac Newton, who copies one of his works by hand - behind every fortune is a crime?

In 1597 German physician-chemist Andreas (Andrew) Libavius (1555-1616) pub. Alchymia, the first systematic chemistry textbook; describes the use of chemistry for drugs, acknowledging the possibility of transmutation; describes how ammonia turns cuprous salt solutions dark blue; first to claim that fermentation and putrefaction are different processes, and to describe a method for distilling alcohol; describes how to make many useful chemicals such as hydrochloric acid and ammonium sulphate.
In 1603 Bolognese alchemist Vincenzo Cascariolo discovers Lapis Solaris, a heated mixture of powdered barite (heavy spar) (barium sulfate) and coal that gives off a bluish glow at night and is recharged by exposure to sunlight, pioneering the study of luminiscence; thinking it's the fabled Philosopher's Stone that turns inferior metals to gold, he starts the myth of the Bologna Stone.
In 1610 French chemist Jean Beguin (1550-1620) pub. Tyrocinium Chymicum (Beginner's Chemistry) in Paris, becoming the first chemistry textbook.

In 1620 Niccolo Cabeo (1586-1650) of Italy discovers that electrified bodies can attract non-electrified ones and that two electrified bodies repel each other.

In 1624 Brussels-born Belgian Flemish chemist Jan (Johannes) Baptist van Helmont (1580-1644) pub. his discovery that the atmosphere is composed of gases, and coins the term "gas" (Gr. "chaos" = unformed) for a compressible fluid - that would make it classical gas?

In 1625 German-Dutch chemist Johann Rudolf Glauber (1604-68) discovers Glauber's Salt (sodium sulfate). In 1648 he discovers (makes) hydrochloric acid.

All great scientific laws are named after people with just the right names? In 1661 English scientist (rival of Isaac Newton) Robert Boyle (1627-91) (after discovering that sound doesn't travel in a vaccum in 1658) pub. New Experiments Physicomechanical, Touching the Spring of the Air (2nd ed.), incl. Proemial Essay, expounding Boil's, er, Boyle's Law (pressure-volume dependence); he stole it from fellow English scientist Richard Towneley (1629-1707), who pub. it in his book "Experimental Philosophy" in 1663 after he saw an early draft in 1661, calling it "Mr. Towneley's Hypothesis". Also in 1661 he uses his pneumatic pump to prove that animals die from lack of air not the accumulation of noxious vapors, stimulating others to begin respiration studies; he also discovers methyl (wood) alcohol - by drinking it? Also in 1661 he pub. The Sceptical Chymist, breaking Chemistry away from Alchemy, dissing the Aristotelian theory of the elements and the Paracelsian theory of principles, and listing chemical elements; "I look upon amity and enmity as affections of intelligent beings, and I have not yet found it explained by any, how those appetities can be placed in bodies inanimate and devoid of knowledge or of so much as sense." In 1666 he pub. Origin of Forms and Qualities According to the Corpuscular Philosophy, explaining his view that everything is mad of atoms, and that Nature is mechanical in er, nature. In 1667 he proves that fresh air is necessary for respiration, and that an animal can be kept alive by artificial respiration using a bellows in a dog's trachea; his partner Robert Hooke shows that blood alteration in the lungs is the essential feature of respiration. In 1679 French physicist-priest Edme (Edmé) Mariotte (1620-84) announces his rediscovery of Boyle's Law - but Edme's Law or Mariotte's Law has no pizzazz?

In 1665 English scientist Robert Hooke (1635-1703) pub. Micrographia, popularizing microscopy; "The most ingenious book that I ever read in my life" (Samuel Pepys); uses the microscope to identify cells, coining the term "cell" for the rigid thingies in cork, which remind him of monks' cells; his 12 in. x 18 in. Drawing of a Flea grosses out sensitive ladies and makes them faint?; he is the first person to examine fossils under a microscope, and concludes they are the remains or traces of long-dead organisms; the microscope is now de rigueur, causing Antony van Leeuwenhoek, Marcello Malpighi, Nehemiah Grew, Jan Swammerdam et al. to bend over and squint - giving future air duct salesmen a job? In 1667 he invents the Anemometer for measuring wind speed, which is also invented the same year by Christian Forner (Förner) of Weissenfels, Germany. On Feb. 5, 1675 Sir Isaac Newton writes a Letter to Robert Hooke, with the soundbyte "If I have seen further it is by standing on ye sholders of Giants"; later when their rivalry goes bitter, Newton starts dropping bigger and bigger slams on Hooke, and ultimately tries to erase his memory? In 1678 he pub. Hooke's Law of Elastic Force ("ut tensio, sic vis", "stress is proportional to strain") - especially on springs with hookes?

In 1733 French chemist Charles Francois de Cisternay du Fay (1698-1739) pub. his discovery that electrical action can be repulsion as well as attraction, calling the two types "vitreous" and "resinous", noting the difference between conductors ("electrics") and insulators ("non-electrics"); he also disproves the theory of Stephen Gray that electric properties of a body depend on its color.

In 1754 Bordeaux-born Scottish chemist Joseph Black (1728-99) discovers carbon dioxide (carbonic acid) (CO2) gas by heating calcium carbonate (CaCO3 ); he calls it "fixed air" because it is denser than air and can't sustain fire or animal life, and discovers its function in the "causticization of lime", which helps disprove the phlogiston theory.

In 1767 Swedish chemist Torbern Olaf Bergman (1735-84) of Uppsala measures and records the first "chemical affinities"; in 1775 he pub. Dissertation on Elective Affinities, containing the largest chemical affinity tables ever pub., first using the A, B, C etc. system for chemical equations.

In 1767 English chemist Joseph Priestley (1733-1804) creates the first artificially carbonated (soda) water; in 1771 Torbern Olaf Bergman of Sweden makes it from chalk and sulfuric acid. In 1770 Priestley discovers sulfur dioxide, and coins the name "rubber" for the substance from an Am. tree that is good for wiping black lead pencil marks from paper - funny how a priest coins the term rubber?

In the 1770s the Chemical Rev. begins in Europe, led by French brain man ("the Father of Modern Chemistry") Antoine-Laurent de Lavoisier (1743-94).
In 1774 Karl Wilhelm Scheele discovers ammonia (NH4), Barium (Ba) (#56), Chlorine (Cl) (#17) and baryta, and, along with English chemist Joseph Priestley (1733-1804) and French chemist Antoine Lavoisier (1743-94) discover the element Oxygen (O) (#16), which is first isolated by Priestley on Aug. 4 by focusing sunlight through a lens onto mercuric oxide (red calx), calling it "dephlogisticated air", finding that breathing it gives him an uplifted sensation, with the soundbyte: "‘The feeling of it in my lungs was not sensibly different from that of common air, but I fancied that my breast felt peculiarly light and easy for some time afterwards"; the belief that there are just four elements (earth, fire, water, and air) is exploded with the discovery that air is a mixture of gases.


In 1776 Swedish chemists Karl (Carl) Wilhelm Scheele (1742-86) and Torbern Olaf Bergman (1735-84) (who originally "discovered" Scheele) independently discover uric acid in kidney stones - good cover story until they're ready to come out of the closet?
In 1779 Antoine-Laurent Lavoisier and Pierre Simon Laplace pub. their discovery that respiration is a form of combustion. In 1779 Scheele discovers Glycerine, and makes the surprising discovery that black pencil lead is actually a soft form of carbon; in 1789 German geologist Abraham Gottlob Werner names it graphite (Gr. graphein = "to write").

In 1780 Italian scientist Felice Fontana (1730-1805) invents the Water Gas Shift (Water Splitting) Reaction, in which H2O and CO react at high temps to form CO2 and H2.




In 1782 French papermaker brothers Joseph-Michel Montgolfier (1740-1810) and Jacques-Etienne (Étienne) Montgolfier (1745-99) of Annonay (near Lyons), France test their first small unmanned hot air balloon, made of paper filled with smoke. Do you know the way to Hot Air Bay? On June 4, 1783 the Montgolfier Brothers publicly demonstrate their paper-lined linen hot air balloon, which rises to a height of 3K ft. at Annonay, France in a 10-min. 1-mi. flight; on Aug. 27 Parisian physicist Jacques Alexandre Cesar (César) Charles (1746-1823) launches a 13-ft.-dia. hydrogen-filled silk balloon (constructed under his supervision by A.J. and M.N. Robert) in front of 50K spectators in Paris; it floats at 3K ft. for more than 45 min. and lands in a village 16 mi. away, where the spooked villagers attack it with stones and knives; on Sept. 19 the Montgolfier Brothers conduct another demo in Versailles, witnessed by Louis XVI and Marie Antoinette, where a duck, rooster, and sheep become the first living passengers, traveling 2 mi. in 8 min., with a max. alt. of 1.5K ft, pissing the king off with the dense smoke they deliberately generate in the belief that it's what causes the buoyancy, later learning that it's heat; on Oct. 15 French daredevil physics teacher Jean Francois Pilatre de Rozier (1754-85) makes the first tethered balloon ascension, repeating the demo on Oct. 17 before a group of scientists, then reaching 324 ft. on Oct. 19; on Nov. 21 (2 p.m.) champagne-toting de Rozier and army officer Francois Laurent Marquis d'Arlandes (1742-1809) make the first untethered human flight, reaching a peak alt. of 500 ft. and traveling 5.5 mi. in 25 min. from the Bois de Boulogne in Paris in the presence of Louis XVI and a huge crowd, across the Seine River to the Butte-aux-Cailles; the next day Benjamin Franklin et al. sign the official certification at Passy; on Nov. 31 Jacques Charles (financed by Franklin) flies in his hydrogen balloon, while gouty Franklin watches from his carriage near the Tuileries Gardens; balloon exhibition flights soon become the rage in Paris; when asked what was the practical use of these balloon thingies, Franklin replies "What is the use of a newborn baby?"; the power of a mere individual to go over the king's head and attract large crowds is a giant leap for popular democracy, making the French Rev. inevitable? - it coulda gone better if only they hadn't dabbled with the powder keg of godless atheism? In 1783 Jacques Charles makes and tests the first hydrogen-filled balloon, which is witnessed by Ben Franklin. In 1787 Charles discovers Charles' Law, describing the relationship between gas volume and temperature.

In 1784 French mineralogist Abbe Rene Just Hauy (Haüy) (1743-1822) of the U. of Paris proposes the Law of Rational Indices, that the regular form of crystals is caused by a regular internal arrangement of tiny cubes or polyhedra ("molecules integrantes"); 174 years later (1958) an electron microscope confirms his model.

In 1786 German scientist Karl Wilhelm Scheele (1742-86) discovers hydrocyanic acid before dying on May 21, and it's no surprise, since he tasted or sniffed all his chemical discoveries, most of which others get credit for, causing Isaac Asimov to call him "hard-luck Scheele"; he actually dies from long-term mercury poisoning?

In 1789 German chemist Martin Heinrich Klaproth (1743-1817) makes the first chemical analysis of Pitchblende, and discovers the element Uranium (U) (#92), which initially is used in ceramics and textiles; he also discovers the metallic element Zirconium (Zr) (#40) in the sands of the rivers of Sri Lanka (Srilankium?); uranium is later used to make fuel elements for atomic reactors, and zirconium in containers for fuel elements since it doesn't absorb neutrons.

In 1789 French chemist ("the Father of Modern Chemistry") Antoine (Antoine-Laurent de) Lavoisier (1743-94) pub. Traite Elementaire de Chimie, the first modern chemical textbook; it defines an element as a single substance that can't be broken down by chemical analysis and from which all chemical compounds are formed; discovers that fermentation produces carbon dioxide (carbonic gas) and spirit of wine, saying that it is "more appropriately called by the Arabic word alcohol since it is formed from cider or fermented sugar as well as wine", and pub. the first chemical equation "grape must = carbonic acid + alcohol", calling it "one of the most extraordinary in chemistry", adding: "In these experiments, we have to assume that there is a true balance or equation between the elements of the compounds with which we start and those obtained at the end of the reaction"; In 1790 he pub. Table of 31 Chemical Elements, founding modern chemistry with the first quantitive chemical experiments, rejecting the phlogiston theory after meticulously burning things and measuring all the byproducts and proving that matter is conserved, considering heat (caloric) and light to be elements and counting the role of oxygen and hydrogen, the components of water, naming both; "Nothing is lost, nothing is created, everything is transformed." In 1790 he is appointed to a committee that develops the Metric System. Too bad, he pisses-off aspiring scientist Jean-Paul Marat, who later pays him back by circulating a denouncement that gets him a free French close shave.

In 1790 French physician-chemist Jean-Antoine Chaptal (1756-1832) pub. Elements of Chemistry (3 vols.) (Montpellier), which coins the term "nitrogen", from Fr. "nitre" (potassium nitrate AKA saltpeter) + "gene" (producing). In 1806 Chaptal pub. La Chimie Appliquee aux Arts, which revolutionizes wine-making in France, describing the Chaptalization process of adding sugar to increase final alcohol content.

In 1790 French surgeon-chemist Nicolas Leblanc (1742-1806) develops a process for producing sodium carbonate (alkali) (used to make lye) from common table salt using sulfuric acid, carbon, and calcium carbonate, paving the way for industrial soap manufacture, but it takes until the end of the 19th cent. for manufactured soap to become popular and have a global market.


In 1799 Italian physicist-chemist Alessandro Giuseppe Antonio Anastasio Volta (1745-1827) makes the shocking discovery of the Voltaic Pile, reporting it to the British Royal Society next year; the first one is made of zinc and copper metal plates and wet cardboard soaked in salt solution, and he later substitutes silver for copper and cloth for cardboard to build bigger piles from which he can draw sparks and shocks, amazing the world and causing a sensation; in May W. Nicholson and A. Carlile use a voltaic pile to decompose water, observing oxygen appearing at one pile and hydrogen at the other, adding to the sensationalism with the idea that atoms are held together by electricity - and hence immortality is just around the corner? In 1818 London-born Mary Wollstonecraft Shelley (1797-1851) pub. the Gothic romance novel Frankenstein, about a mad scientist who makes a corpse live again via electricity; she got the idea while in a trance based on the writings of alchemists about creating a homunculus in a test tube, "a pale student of the unhallowed arts [grave-robbing] kneeling beside the thing he had put together"; "I beheld the wrath of the miserable monster whom I had created"; "I curse (although I curse myself) the hands that formed you" - could it have really been based on her hubby Percy's anatomy?

In 1801 German chemist Johann Wilhelm Ritter (1776-1810) and English chemist-physicist William Hyde Wollaston (1766-1828) independently discover Ultraviolet Light beyond the violet - now the nightclubs will rock?

In 1809 French scientist Etienne-Louis Malus (1775-1812) pub. his discovery of the polarization of light by reflection; in 1810 he pub. his theory of double refraction of light in crystals.

In 1811 Turin-born Italian scientist Count Lorenzo Romano Amedeo Carlo Avogadro (1776-1856) proposes Avogadro's Principle (Law), that equal volumes of gases contain equal numbers of molecules (not atoms) at a given temperature and pressure; his ideas are neglected until 1858?
In 1811 English chemist Humphry Davy discovers colorless poisonous heavy gas Phosgene (carbonyl chloride).


In 1811 French chemist-physicist Pierre Louis Dulong (1785-1838) discovers the double decomposition of salts. In 1812 he discovers sensitive unstable nitrogen trichloride, losing two fingers and an eye. In 1817 he discovers the oxides of phosphorus. In 1819 Dulong and French physicist Alexis Therese (Thérèse) Petit (1791-1820) of France pub. the Law of Dulong-Petit, that the heat capacity of a mole of a solid element is about 3R, where R is the universal gas constant, i.e., that relative atomic weight is inversely proportional to specific heat, i.e., that approximately the same amount of heat is required to accomplish a particular rise in temperature of the same number of atoms of any metal, making possible the experimental determination of atomic weight - du long and winding road to specific petit?

In 1812 German-born Russian chemist Gottlieb Sigismund Constantin Kirchhoff (1764-1833) obtains glucose (Gr. for "sweet") (AKA corn syrup) by treating starch with sulfuric acid, becoming an early use of a catalyst.


The original mad scientists start out as pop stars? In 1812 English scientist pop star Humphry Davy (1778-1829) (the original Davy Jones?) is knighted and becomes Sir Humphry Davy; he permanently damages his eyes in a nitrogen chloride explosion this year, and his reckless experiments and love of sniffing laughing gas keep him an invalid to his death in 1829; his misfortune is the luck of Michael Faraday (1791-1867) (the original Robin?), as the eye injury causes Davy to hire him as his secy. and lab asst. next year, where he matures into his protege and rival ("his best discovery"), and invents (with William Whewell) the cool new nomenclature for electric junkies, such as electrode, electrolysis, electrolyte, anion (electrode toward which anions move), cation, and ion; unfortunately Faraday also injures his eyes in a nitrogen chloride explosion, and goes on to suffer from chronic chemical poisoning.

In 1823 Michel Eugene Chevreul (1786-1889) pub. Researches on Animal Fats (Recherches sur les corps gras d'origine animale); first analysis of soap, incl. stearin (white solid substance), olein (liquid fat), and oleic acids, giving them their names; he goes on to discover margaric acid and creatine, pioneer the field of gerontology, and become one of the 72 scientists and engineers inscribed on the Eiffel Tower, dying 10 days after Gustave Eiffel plants the French Tricolor on top, becoming the last person born before the French Rev. to die.

In 1829 German chemist Johann Wolfgang Dobereiner (Döbereiner) (1780-1849) pub. the existence of a simple relationship among the atomic weights of elements having similar properties - he's got the columns but not the rows?



In 1831 Samuel Guthrie (1782-1848) of N.Y. (inventor of percussion powder, which makes the flintlock musket obsolete, plus a process for converting potato starch into molasses in 1830), Justus von Liebig (1803-73) of Germany, and Eugene (Eugène) (1797-1859) of France independently discover Chloroform (CHCl3). In 1839 Soubeiran and Hyacinthe Captaine discover Cubebin.

In 1838 Dutch chemist Fox Mulder, er, Gerardus Johannes Mulder (1802-80) finds nitrogen and sulfur atoms in albuminoids, and decides that their structure is more complex than carbohydrates and lipids, causing Jons Jakob Berzelius to suggest the name "protein", from the Greek word for "of primary importance".
In 1839 Ozone (O3) (gr. "ozein" = to smell) is discovered and named by German-Swiss scientist Christian Friedrich Schoenbein (Schönbein) (1799-1868), co-inventor of the fuel cell in 1838; its molecular structure and formula is determined in 1865 by Swiss chemist Jacques-Louis Soret (1827-90), which are confirmed by Schoenbein in 1827.

In 1840 Swiss-German chemist Germain Henri Hess (1802-50) pub. Hess' Law of Constant Heat Summation, that the total energy (enthalpy) gained or lost in a series of chemical reactions depends only on the initial and final statements, becoming a progenitor of the First Law of Thermodynamics; in 1842 he proposes the law of thermoneutrality, that exchange reactions of neutral salts in aqueous solutions evolve no heat.

In 1842 Robert Wilhelm Eberhard von Bunsen (1811-99) of Germany invents the carbon electrode battery, far less expensive than William Robert Grove's platinum electrode battery. Too bad, in 1843 he loses the use of his right eye in a cacodyl cyanide explosion, and, remembering two near-deaths from arsenic poisoning, he decides to switch from organic to inorganic chemistry.

In 1855 German chemist Robert Wilhelm Eberhard Bunsen (1811-99) invents the gas Bunsen Burner, and magnanimously refuses to file a patent application; actually technician Peter Desaga invents it but he takes the credit?


In 1858 German chemist Friedrich August Kekule (Kekulé) von Stradonitz (1829-96) and Scottish chemist Archibald Scott Couper (1831-92) independently pub. the theory of chemical bonds, suggesting that carbon is tetravalent, and that carbon-carbon bonds are the key structural feature of organic compounds, becoming the key insight that gets modern organic chemistry cooking? In 1865 von Stradonitz develops Benzene Ring Theory to explain the properties of aromatic compounds, based on a dream about a snake swallowing its own tail.

In 1859 Heidelberg U. profs. Robert Wilhelm Eberhard Bunsen (1811-99) (1855 inventor of the Bunsen Burner) and Gustav Robert Kirchhoff (1824-87) team up and begin experimenting with spectrum analysis after Kirchhoff's Three Laws of Spectroscopy is announced, that if a body absorbs light of a certain wavelength, it emits the same wavelength (thus the dark Fraunhaufer lines in the solar spectrum are caused by elements in the Sun absorbing various wavelengths, and if one can find elements on Earth that give bright emission lines at these same wavelengths, the composition of the Sun can be deduced without having to visit it and vaporize first). In 1860 they discover the elements Cesium (Cs) (#55) (most electropositive element) and Rubidium (Rb) (#37), both of which ignite spontaneously in air, becoming the first time that spectroscopic identification is used to prove the existence of new elements; their book Chemical Analysis by Observation of Spectra causes spectral analysis to become an instant sensation.

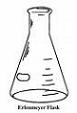
In 1860 German chemist Richard August Carl Emil Erlenmeyer (1825-1909) invents the conical Erlenmeyer Flask. In 1880 he formulates the Erlenmeyer Rule, that alcohols which have a hydroxyl group directly attached to a double-bonded carbon atom become aldehydes or ketones.


In 1861-5 the horrific U.S. Civil War sees the invention of the first modern weapon when N.C.-born agricultural equipment maker Richard Jordan Gatling (1818-1903) patents the 10-barrel hand-cranked hundreds-of-rounds-per-min. Gatling Gun (the first practical machine gun) just in time for use on some Johnny Rebs; it is first used by the Union Army in 1864, but luckily never sees extensive use.

In 1862 German botanist Julius von Sachs (1832-97) discovers that starch is produced by photosynthesis. In 1865 he discovers that chlorophyll in plants is found only in small bodies (later termed chloroplasts), and that chlorophyll is the key compound in combining carbon dioxide and oxygen to produce starch while releasing more oxygen.

In 1863 English chemist John Alexander Reina Newlands (1837-98) (who just returned from fighting for Giuseppe Garibaldi in Italy) pub. the first Periodic Table of the Elements arranged in order of relative atomic masses, leaving open the possibly of undiscovered elements, and predicting the existence of germanium, scooping Dmitri Mendeleyev of Russia, although he is ridiculed.

In 1865 Belgian scientist Jean Servais Stas (1813-91) produces the first modern table of atomic weights, using oxygen as the index (set at 16), and shows conclusively that atomic weights are not always integral.


On Mar. 1, 1869 (Feb. 17 Old Style) Russian chemist Dmitri Ivanovich Mendeleyev (Mendeleev) (1834-1907) pub. the Periodic Law and the Periodic Table of the Elements, which future chemistry students are face-forked with, boldly making room for the missing element gallium; its appearance is influenced by the new game of Solitaire? - so, village atheist, if everything happened by chance, and there is no intelligent designer, why is there a mathematical code behind matter itself? In 1871 Mendeleyev pub. The Periodic Regularities of the Chemical Elements, which boldly predicts that some accepted atomic weights are grossly in error, and other elements yet to be discovered will plug up gaps in his Periodic Table. Which chemical element is named for a cock? In 1875 using zinc blende from the Pyrenees, French chemist Paul-Emile (Francois) Lecoq de Boisbaudran (1838-1912) discovers the metallic element Gallium (#31) (Ga), which resembles aluminum but can be cut with a knife (melting point 30.15 deg C), right where Mendelyeev had predicted an element with these properties would be found in a gap in the Periodic Table; he suggests the name "eka aluminum" for it, but the name gallium is chosen as a pun on the Latin word gallus (cock).

In 1869 Russian chemist Vladimir Vasilyevich Markovnikov (Markownikoff) (1838-1904) develops the "rich get richer, poor get poorer" Markovnikov's (Markownikoff's) Rule, which states that in reactions of alkenes (olefins) (molecules containing a carbon-carbon double bond) and vinyl halides (H-X), the nucleophilic halide X group tends to attach to the carbon atom in the double bond with the fewest hydrogen atoms bonded to it, while the acidic proton H tends to attach to the carbon atom in the double bond with the most hydrogen atoms attached to it, e.g., CH3-CH=CH2 + HBr -> CH3CBrHCH3, because the left C has 1 H and the right C has 2 Hs (the CH3 is irrelevant); the rule extends to reactions of an alkene with an alcohol, with the hydroxyl (OH) group bonding to the C in the double bond with the greatest number of C-C bonds, and the H bonding to the C at the other end of the double bond.

In 1869 Swiss biologist-physician Johannes Friedrich Miescher (1844-95) discovers a weak acid in white blood cell nuclei, which he calls nuclein; in 1871 he isolates it; it turns out to be nucleic acid (DNA); he later discovers that CO2 concentrations in blood regulate breathing.


In 1874 Dutch physical chemist Jacobus Henricus van't Hoff (1852-1911) and French physical chemist Joseph Achille Le Bel (1847-1930) independently suggest that the four bonds in most carbon compounds are directed toward the corners of a tetrahedron, thus founding the study of Stereochemistry, and winning van't Hoff the first-ever Nobel Chem. Prize in 1901.
In 1874 German physicist Friedrich Wilhelm Georg Kohlrausch (1840-1910) demonstrates that an electrolyte has a definite constant value of electrical resistance, and goes on to to verify the independent migration of ions.

In 1875 Am. chemist-physicist ("Founder of Chemical Thermodynamics") Josiah Willard Gibbs (1839-1903) of Yale U. pub. the Gibbs Phase Rule, applicable to the phases of water, carbon dioxide, etc.: f = c - p + 2, i.e., number of degrees of freedom (number of intensive state variables that can be independently varied without changing the number of phases) equals the number of components - number of phases + 2; thus, for a 1-component system, f = 3 - p, and if there is just one phase in equilibrium it takes a 2-dim. graph to describe it (P vs. T, P vs. V), if there are two it takes a 1-dim. "tie line", and for three phases in equilibrium there is a 0-dim. "critical point".

In 1877 French chemist Charles Friedel (1832-99) and Am. chemist James Mason Crafts (1839-1917) pub. the Friedel-Crafts Reaction for attaching alkyl and other groups to an aromatic ring using the chloride of aluminum as catalyst, becoming one of the most fruitful synthetic methods in organic chemistry, allowing hundreds of new carbon compounds to be created.
In 1883 German biochemist Ludwig Karl Martin Leonhard Albrecht Kossel (1853-1927), teacher of Friedrich Miescher at the U. of Strasbourg moves to the U. of Berlin, going on to isolate the five constituent organic compounds in nucleic acid incl. adenine, cystosine, guanine, thymine, and uracil; he later discovers histidine and isolates theopylline from tea, and predicts the polypeptide nature of the protein molecule; "The processes of life are like a drama, and I am studying the actors, not the plot. There are many actors, and it is their characters which make this drama. I seek to understand their habits, their peculiarities."

In 1884 Swedish chemist Svante August Arrhenius (1859-1927) proposes the ionic theory of chemistry in his doctoral dissertation, pointing out that solutions of salt in water are better conductors than pure salts or pure water, and dissociation into ions is practically complete even when there is no passage of electric current; his teachers barely pass him, but he later wins the 1903 Nobel Chem. Prize.


In 1886 German chemist Friedrich Otto Schott (1851-1935) and German microscope maker Carl Zeiss (1816-88) invent a new type of optical glass that can utilize the Abbe sine condition, making possible apochromatic (apo) microscope objectives, which bring three wavelengths into focus in the same plane rather than two like achromatic lenses. In 1887-93 Schott develops borosilicate glass (ends 1893), which can take heat and thermal shock and resist chemicals. In 1908 Eugene Sullivan of Corning Glass Works develops Nonex borosilicate glass for battery jars and lantern globes; in 1913 Pyrex ("pie" as in pie plate + "ex") brand glass is invented by Jesse Littleton of Corning when he uses a cut-down Nonex battery jar for a casserole dish; it is introduced commercially in 1915 to compete with the Duran borosilicate glass of Schott AG in Germany.

In 1887 German chemist Rainer Ludwig Claisen (1851-1930) pub. the Claisen Condensation, a reaction between two esters or one ester and a carbonyl compound (activated methylene group) in the presence of a strong base, yielding a beta-keto ester or a beta-diketone after forming a carbon-carbon bond. In 1890 he pub. the Claisen Reaction, the synthesis of cinnamates by reacting aromatic aldehydes with esters; in 1912 he discovers the Claisen Rearrangement of allyl phenyl ether by heat, becoming the first known [3,3]-sigmatropic rearrangement.

In 1887 German chemist Friedrich August "Fritz" Raschig (1863-1928) develops the Raschig Process for production of hydrazine from organic compounds via oxidation of ammonia with sodium hypochlorite.

In 1890 German chemist Julius Wilhelm Theodor Curtius (1857-1928) pub. the Curtius Rearrangement, the thermal decomposition of an acyl azide to an isocyanate with the loss of nitrogen, going on to produce azoimide (hydrogen azide) (hydrazoic acid) from organic sources.

In 1895 Russian-Latvian-German chemist Paul Walden (1863-1957) discovers Walden Inversion, the first Stereoinversion Reaction; in the early 1900s Christopher Ingold finds that it doesn't work with tertiary alcohols, which is solved in the Sept. 12, 2013 issue of Nature by Ryan A. Shenvi et al. of the Scripps Research Inst.


In 1897 German chemist Eduard Buchner (1860-1917) observes bubble formation in sugar-filled yeast extract in experiments of his bacteriologist brother Hans Ernst August Buchner (1850-1902), and breaks up yeast cells with hundreds of atmospheres of pressure, fine quartz sand, and filter paper and still obtains fermentation, laying to rest Pasteur's theory that only living yeast cells can do it, discovering zymase, the first enzyme, and later receiving the 1907 Nobel Chem. Prize.

In 1900 French chemist Francois Auguste Victor Grignard (1871-1935) pub. the Grignard Reaction, using magnesium-containing Grignard reagants to add a carbonyl group to an aldehyde or ketone, forming carbon-carbon bonds, winning him the 1912 Nobel Chem. Prize.

In 1900 German chemist Friedrich Wilhelm Ostwald (1853-1932) discovers the Ostwald-Brauer Process (patented in 1902) for oxidizing ammonia to prepare nitric acid for explosives, which Germany uses in WWI to beat the Allied blockade of nitrates.

In 1906 English biochemist Arthur Harden (1865-1940) discovers cases of catalysis among enzymes, going on to win the 1929 Nobel Chem. Prize.

In 1906-12 German chemist Walter Hermann Nernst (1864-1941) formulates the Third Law of Thermodynamics.

In 1908 German chemist Hermann Emil Louis Fischer (1852-1919) discovers Peptide Chains, consisting of amino acids that fold in three dims. to produce proteins.

In 1908 Japanese scientist Kikunae Ikeda (1864-1936) discovers L-glutamic Acid, the active ingredient in kombu seaweed, whose taste he calls umami, causing its salt monosodium glutamate (MSG) to begin to be used as a flavor enhancer; during WWII the U.S. finds out about it, and it is introduced to the U.S. food industry after the war.

About 1910 French chemist Paul Sabatier (1854-1941) discovers the Sabatier Process (Reaction), the reaction of hydrogen with carbon dioxide at high temps (300C-400C) and high pressure in the presence of a nickel catalyst to produce methane and water, winning him the 1912 Nobel Chem. Prize.



In 1911 Polish chemist Casimir (Kazimierz) Funk (1884-1967) coins the term "vitamine" (changed to vitamin in 1920) after reading an article by Dutch physician Christiaan Eijkman (1858-1930) claiming that people who eat brown rice are less vulnerable to beriberi than those who eat white race, er, rice, causing him to isolate vitamin B1, which has an amine group, cogito ero sum; meanwhile English biochemist call-me-sir Frederick Gowland Hopkins (1861-1947) goes on to conduct experiments which discover other vitamins, gaining him and Eijkman the 1929 Nobel Med. Prize.


In 1911 German chemists Fritz Haber (1868-1934) and Carl Bosch (1874-1940) develop the Haber-Bosch Process for synthesizing ammonia on an industrial scale from hydrogen and air, freeing the production of fertilizer and explosives from natural ammonia deposits such as sodium nitrate (calich) (which is monopolized by Chile), and averting global famine, winning Haber the 1918 Nobel Chem. Prize; too bad, brainy Jew Haber, who coulda been a contender stinks himself up by plunging into poison gas research, finding a way to turn science from good to bad - break out my quills I'm going to wax poetic?

In 1912 Danish chemist Niels Bjerrum (1879-1958) pub. On the Infared Spectra of Gases showing that infared absoption by molecules is caused by uptake of rotational and vibrational energy in definite quanta, becoming the first correct application of quantum theory to interpretation of spectra.


In 1912 German Merck chemist Anton Kollisch (Köllisch) (1888-1916) synthesizes MDMA (Ecstasy) (AKA Mandy, Molly) to stop abnormal bleeding; it is patented on May 16, 1914; its potential as a recreational drug is first publicized in 1978 by Am. chemist Alexander Theodore "Sasha" Shulgin (1925-2014) of UCB, who compares it "to marijuana, and to psilocybin devoid of the hallucinatory component", causing the drug's popularity to take off, and Shulgin to become known as "the Godfather of Psychedelics"; Shulgin calls it "my low-calorie martini".

In 1912 French chemist Louis Camille Maillard (1878-1936) pub. the Maillard Reaction between the carbonyl group of sugar and the nucleophilic amino group of amino acid at 140C-165C that gives browned food a desirable flavor.

In 1912 English radiochemist Frederick Soddy (1877-1956) of the U. of Glasgow coins the term "isotope".

In 1913 German chemist Friedrich Karl Rudolf Bergius (1884-1949), student of Fritz Haber and Carl Bosch develops the Bergius Process, which hydrogenates lignite coal to produce oil for use as synthetic fuel, winning him the 1931 Nobel Chem. Prize; he later develops a process for converting wood into sugar.

In 1913 German physical chemist Max Ernst August Bodenstein (1871-1942) formulates the concept of the chemical chain reaction - that is what makes this country dynamic?

In 1913 Harvard chemist-biologist Lawrence Joseph Henderson (1878-1942) pub. The Fitness of the Environment: An Inquiry into the Biological Significance of the Properties of Matter, "an inquiry into the biological significance of the properties of matter", becoming the first major book to develop the concept of fine tuning in the Universe; "The properties of matter and the course of cosmic evolution are now seen to be intimately related to the structure of the living being and to its activities; they become therefore, far more important in biology than has been previously suspected. For the whole evolutionary process, both cosmic and organic, is one, and the biologist may now rightly regard the universe in its very essence as biocentric"; "One of the remarkable physical properties of carbon dioxide is its degree of solubility in water. This quality varies enormously in different substances. For example, at ordinary pressures and temperatures, water can absorb only about 5 per cent of its own volume of oxygen, while it can take up about 1300 times its own volume of ammonia. Now for carbon dioxide, unlike most gases, the volume that can be absorbed by water is nearly the same as the volume of the water. The volumes vary, however, according to temperature, being absolutely the same at a temperature of about 15ºC. or 59ºF., which is close to the ideal temperature for man's physical health and practically the same as the mean temperature of the earth's surface when all seasons are averaged together"; "Hence, when water is in contact with air, and equilibrium has been established, the amount of free carbonic acid in a given volume of water is almost exactly equal to the amount in the adjacent air. Unlike oxyzen, hydrogen, and nitrogen, carbonic acid enters water freely; unlike sulphurous oxide and ammonia, it escapes freely from water. Thus the waters can never wash carbonic acid completely out of the air, nor can the air keep it from the waters. It is the one substance which thus, in considerable quantities relative to its total amount, everywhere accompanies water. In earth, air, fire, and water alike, these two substances are always associated"; "Accordingly, if water be the first primary constituent of the environment, carbonic acid is inevitably the second, - because of its solubility possessing an equal mobility with water, because of the reservoir of the atmosphere never to be depleted by chemical action in the oceans, lakes, and streams. In truth, so close is the association between these two substances that it is scarcely correct logically to separate them at all; together they make up the real environment and they never part company."



In 1913 Yale U. biochemists Thomas Burr Osborne (1859-1929) and Lafayette Benedict Mendel (1872-1935) isolate fat-soluble Vitamin A in butterfat and water-soluble Vitamin B in milk; discovered independently by U. of Wisc. biochemists Marguerite Davis (1887-1967) and Elmer Verner McCollum (1879-1967) (known for establishing the first colony of white lab rats in the U.S.), who gives them the letter names and refuses to their being called vitamins (vitamines) because they're not any more vital than other nutrients and are not true amines; he later refuses to take vitamin supplements; in 1922 he discovers Vitamin D.

In 1913 German organic chemist Richard Willstatter (Willstäter) (1872-1942) uses chromatography to discover the composition of chlorophyll.





On Aug. 4, 1914 - Nov. 11, 1918 the horrific World War I causes 15M deaths and 39M military casualties. and destroys the Old Order of white formerly Christian Europe. On Feb. 26-28, 1915 the Germans first use a Flamethrower (Flame Projector) in the village of Douaumont, France near Verdun, becoming the first of 653 flamethrower attacks in the war. On Apr. 1, 1915 French aviator Roland Garros (1888-1918) becomes the first pilot to shoot down an aircraft using a deflector gear, which allows shooting through the propeller; after more Vs against German aircraft on Apr. 15 and Apr. 18, he is shot down and the Germans capture his plane, after which Dutch designer Anthony (Anton Herman Gerard) Fokker (1890-1939) clones then improves the deflector gear into the synchronization (interrupter) gear, mounting them on the new Fokker E.I. in Aug., beginning the Fokker Scourge (Scare) as they shoot down nearly every enemy aircraft they encounter and generate the first German aces, incl. Max Immelmann (1890-1916); next year the French counter with the Nieuport 11 Bebe (Bébé), in which the gun is mounted on the top wing clear of the prop, and the British with the Royal Aircraft Factory F.E.2b and Airco DH.2 (Feb. 1916), which mount the engine backwards with the prop in back, causing them to be called "pushers", ending the Fokker Scourge by spring 1917. In 1915 arsenic-based vomiting-sneeze gas Adamsite (DM) (diphenylaminechlorarsine) is synthesized by German chemist Heinrich Otto Wieland (1877-1957); in 1918 Am chemist Roger Adams (1889-1971) duplicates it, and both sides stockpile it, but it is allegedly never used on the battlefield. On Mar. 22, 1916 the British have their first success with their new Depth Charge off the SW coast of Ireland, destroying a German U-boat. Are you used to Hell yet, try this? On Sept. 15, 1916 Winston Churchill's pet project the Tank (Russian Water Closet) (Char-Schneider) is first used by the Brits in the Somme.

In 1915 Russian-born British Jewish chemist Chaim Azriel Weizmann (1874-1952) (first pres. of Israel in 1949-52) invents the ABE (Acetone-Butanol-Ethanol) Process, which extracts acetate from British chestnuts for production of the low-grade explosive Cordite, used as a propellant in explosive shells, replacing distillation of birch, beech, and maple wood, which is only available in large enough quantities in Germany, Austria, Canada, and the U.S., leaving Britain out of luck in WWI until manufacturing is cranked up in Feb. 1916.




In 1916 Am. chemist Gilbert Newton Lewis (1875-1946) pub. his Lewis Dot Structures, founding modern valence (covalent bond) theory, which is expanded on by Irving Langmuir (1881-1957), who in 1919 pub. The Arrangement of Electrons in Atoms and Molecules, proposing the concentric theory of atomic structure and defining the concept of valence shells. In 1923 Danish physical chemist Johannes Nicolaus Bronsted (Brønsted) (1879-1947) and English physical chemist Thomas Martin Lowry (1874-1936) independently pub. the Bronsted-Lowry Theory of Acids and Bases, defining an acid as a compound tending to give up a proton, and a base as one tending to take one up. In 1923 Lewis and Langmuir pub. Thermodynamics and the Free Energy of Chemical Substances, which founds modern chemical thermodynamics?


In 1918 Canadian-Am. physicist Arthur Jeffrey Dempster (1886-1950) develops Mass Spectrometry, followed next year by English chemist-physicist Francis William Aston (1877-1945) (J.J. Thomson's asst.), who constructs the first Mass Spectograph, which uses electromagnetic fields to bring particles of the same mass to a focus at the same fine line, and uses it to verify the whole number rule, which states that all atomic weights are integers, and that fractional atomic weights are due to the presence of two or more isotopes, each of which has an integral atomic weight; he goes on to improve its resolution 20x by 1937, winning the 1922 Nobel Physics Prize.

In 1922 Czech chemist Jaroslav Heyrovsky (1890-1967) of Charles U. in Prague invents Polarography during investigations of the electrode potential of aluminum, pioneering the electroanalytic method and the field of Electrochemistry, winning him the 1959 Nobel Chem. Prize.

In 1922 Vitamin E (antisterility factor X) (alpha-tocopherol) is discovered by Am. embyrologist Herbert McLean Evans (1882-1971) and his asst. Katherine S. Bishop of the U. of Calif.; named by E.V. Shute in 1924.


In 1923 Dutch physical chemist Peter Debye (1884-1966) and German physical chemist Erich Armand Arthur Joseph Huckel (Hückel) (1896-1980) of Germany extend the Arrhenius Theory of Ionization of salt in solution to the crystalline solid state, producing the Debye-Huckel Theory of electrolyte ionization to aid in the calculation of activity coefficients.

In 1923 Swedish chemist Theodor H.E. "The" Svedberg (1884-1971) develops the use of the 1M g Ultracentrifuge in distinguishing proteins from each other, winning the 1926 Nobel Chem. Prize.


In 1928 German chemists Otto Paul Hermann Diels (1876-1954) and Kurt Alder (1902-58) pub. their discovery of the Diels-Alder Reaction for diene synthesis of complex aromatic organic ring compounds and plastics - more ways Dirt Devil helps you fight dirty?

In 1930 Am. biochemist John Howard Northrop (1891-1987) crystallizes the enzyme pepsin, and shows that it is a protein; he does the same with trypsin in 1932, and chymotrypsin in 1935; it is later found that all enzymes are proteins, and he wins the 1946 Nobel Chem. Prize for it.

In 1930 Am. chemist Ernest Henry Volwiler (1893-1992) et al. of Abbott Laboratories discover the short-acting barbituate Pentobarbital, which becomes a favorite for execution of convicts; on Mar. 8, 1934 after being discovered by Volwiler et al., physician Ralph M. Waters begins clinical tests of the fast-acting gen. anesthetic Sodium Thiopental (Pentothal), which becomes the anesthetic of choice to initiate surgery, as well as a "truth serum" and legal injection.

In 1931 Adolf Friedrich Johann Butenandt (1903-95) of Germany isolates Androsterone, the first crystalline male hormone, later discovered to be a Pheromone - Adolph's butt hunt? In 1934 he synthesizes the male hormone testosterone, produced by male testes - whinny?

In 1931 Moscow-born Swiss chemist Paul Karrer (1889-1971) of the U. of Zurich isolates Vitamin A, found in carrots - what's up, Doc? In 1935 he synthesizes Riboflavin (Vitamin B2). He shares the 1937 Nobel Chem. Prize with Walter Haworth.

In 1931 Am. physical chemist Harold Clayton Urey (1893-1981) discovers the hydrogen isotope Deuterium (Gk. "deuteros" = second) (heavy hydrogen, incl. a neutron in the nucleus), winning him the 1934 Nobel Chem. Prize.

Everybody can own a pic of Edwin-burgh? In 1932 Am. chemist Edwin Herbert Land (1909-91) invents Polaroid Glass, the first practical synthetic light-polarizing material. On Feb. 21, 1947 he publicly demonstrates his Polaroid Land Camera, which develops its own B&W photo in 60 sec.; it takes until 1962 to go color.

In 1932 chemists Fritz Mietzsch (1896-1958) and Josef Klarer (1898-1953) of Bayer Co. in Germany patent Sulfa Drugs (sulfonamides), the first effective antiobiotic and first bioactivated medicine; too bad, they lose their patent because the active molecule sulfonilamide was discovered in 1906, causing a boom in usage; a team led by physician Gerhard Johannes Paul Domagk (1895-1964) of Bayer Co. in Germany discovers Prontosil, the first sulfa drug for treating streptococcal infections, and the first commercial antiobiotic, winning the 1939 Nobel Med. Prize; English physician Leonard Colebrook (1883-1967) introduces it as a cure for puerperal fever.

On Mar. 8, 1934 after being discovered by Am. chemist Ernest Henury Volwiler (1893-1992) et al. of Abbott Laboratories, physician Ralph M. Waters begins clinical tests of the fast-acting gen. anesthetic Sodium Thiopental (Pentothal), which becomes the anesthetic of choice to initiate surgery, as well as a "truth serum" and legal injection.

In 1935 Am. biochemist William Cumming Rose (1887-1985) discovers threonine, the last of the 20 amino acid molecules in proteins; the first were discovered in 1820.

In 1935 Am. Dupont chemist Wallace Hume Carothers (1896-1937) invents Nylon thread by squeezing a chemical solution through a hypo needle; it is originally known as Polymer 66, renamed after the main Dupont HQs in NY and London, and patented in 1937, but deliberately not trademarked - they don't want people to call it humeon or carotheron? On Feb. 24, 1938 the first nylon products, toothbrushes are marketed in N.J. by Dupont, which doesn't announce the name "nylon" for its new synthetic yarn until Oct. 27. On Oct. 24, 1939 nylon stockings are sold publicly for the first time in Wilmington, Del.

In 1937 German-born English Jewish biochemist Hans Adolf Krebs (1900-81) identifies the 8-step Citric Acid (Krebs) Cycle, the metabolic pathway for cellular oxidation of carbohydrates and fats.




In 1938 German radiochemists Otto Hahn (1879-1968) and Friedrich Wilhelm "Fritz" Strassmann (1902-80) bombard uranium with neutrons and find a barium isotope in the product; Lise Meitner (1878-1968) and her nephew Otto Robert Frisch (1904-79) (Jews) explain the result by fission of the uranium nucleus, with the mass difference accounted for by Einstein's formula E=MC^2, and nuclear fission is a reality; Hahn wins the 1944 Nobel Chem. Prize, becoming known as "the Father of Nuclear Chemistry"; Meitner is snubbed; Niels Bohr goes on to develop the theory of fission, and is the first to point out that U-235 is responsible for observed fission phenomena; meanwhile Lisa Meitner escapes from Berlin to Copenhagen with the help of Dutch physicist Dirk Coster, then goes to Sweden - the Allies won WWII right there?

In 1942 Am. chemists Harold Wesley "Harry" Coover Jr. (1917-2011) and Fred Joyner of Kodak Labs. invent Cyanoacrylate by accident while trying to develop an optically clear plastic for gunsights; in 1951 they rediscover it, along with its adhesive properties, creating Eastman 910 AKA Super Glue in 1958.


In 1942 Am. physician George Herbert Hitchings (1905-98), and Am. biochemist Gertrude Belle Elion (1918-98) of Wellcome Research Labs begin working on chemotherapy drugs, going on to develop pyrimethamine for malaria, thioguanine and 6-mercaptopurine for leukemia, allopurinol for gout, azathioprine for organ transplanation, and co-trimoxazole for bacterial infections, leading the way to research for antiviral drugs for herpes (acyclovir) and AIDS (zidovudine), winning them the 1988 Nobel Med. Prize.
In July 1948 French chemist Jacques (Jack Arthur) Masquelier pub. his discovery of Oligomeric Proanthocyanidins (OPCs), a flavonoid found in grapes, apples, red wine, berries, cocoa beans, cinnamon bark, and tea in peanut skins; OPCs are later linked to reduced risk of coronary heart disease and lower mortality.


Chemistry evolves from mere mathematical formulas to the odd hassle of 3-D diagrams? In 1950 English chemist Sir Derek Harold Richard Barton (1918-98) shows that organic molecules can be assigned a preferred conformation based on the work of Norwegian chemical physicist Odd Hassel (1897-1981), founding Conformational Analysis, winning them the 1969 Nobel Chem. Prize.

In 1950 Austrian-born Am. biochemist Erwin Chargaff (1905-2002) of Columbia U. pub. Chargaff's Rule 1, that the number of adenine and thymine bases, and the number of cytosine and guanine bases are equal to each other, surmising that "they could very well serve as one of the agents, or possibly the agent, concerned with the transmission of inherited properties"; he also proves Chargaff's Rule #2 that the composition of DNA varies between species.



In 1950 motorcycle-loving English geneticist Oliver Smithies (1925-) discovers Gel Electrophoresis, a technique for separating protein molecules using an electric current applied to a starch gel matrix; actually, sucrose was used for it way back in the 1930s, but this process works better.


In 1953 the light-independent carbon-fixation Calvin (Calvin-Benson) (Calvin-Benson-Bassham) Cycle is discovered by UCB chemists Melvin Ellis Calvin (1911-97), James Alan Bassham (1922-2012), and Andrew Alm Benson (1917-2015), explaining the path of carbon in photosynthesis, winning Calvin the 1961 Nobel Chem. Prize.

In 1954 West German chemist Georg Wittig (1897-1987) discovers the Wittig (Olefination) Reaction of an aldehyde or ketone with a Wittig reagant (triphenyl phosphonium ylide) to yield an alkene and a triphenylphosphine oxide, winning him the 1979 Nobel Chem. Prize.

In 1955 Spanish-born Am. biochemist Severo Ochoa de Albornoz (1905-93) pioneers RNA synthesis, winning the 1959 Nobel Med. Prize.

In 1955 Scottish biochemist Sir Alexander Robertus Todd (1907-97) determines the chemical makeup of nitrogenous bases, synthesizing adenosine triphosphate (ATP) and flavin adenine dinucleotide (FAD); he also determines the structure of Vitamin B12, going on to do ditto for Vitamin B1 and Vitamin E, contributing to the scientific knowledge of nucleotides and nucleotide co-enzymes and winning the 1957 Nobel Chem. Prize.

In 1956 Am. biochemist Arthur Kornberg (1918-2007) pioneers DNA synthesis with enzymes and nucleotides, winning him the 1959 Nobel Med. Prize.

In 1956 Chinese-born biochemist Choh Hao Li (1913-87) of the U. of Calif. Medical Center in San Francisco first isolates and purifies Human Growth Hormone (HGH) (Somatotropin), going on to discover its structure in 1966 and synthesize it in 1970 - ask Hao?

In 1957 Tang brand powdered orange drink is developed in Brooks City, Tex. for NASA by Am. food chemist William A. "Bill" Mitchell (1911-2004) of Gen. Foods Corp.; it is first marketed in 1959; sales are poor until it is used on John Glenn's Mercury flight in Feb. 1962; Mitchell goes on to develop quick-set Jell-O, Cool Whip, powdered egg whites, and Pop Rocks astronauts get all the tang they want on the ground?

In 1958 Scottish scientist Sir James Whyte Black (1924-2010) develops Inderal (Propranolol), the first successful beta blocker for treatment of hypertension, which is approved by the U.S. FDA in 1976. In 1976 SmithKline's revolutionary gastric acid inhibitor Tagamet (Cimetidine), developed by Black is first marketed in Britain; Black is awarded the 1988 Nobel Medicine Prize for it and Propanolol; the U.S. FDA approves it on Aug. 23, 1977 after 200K patients in the U.K., Canada, and Mexico receive it.


In 1959 Vienna-born Cambridge U. I-smell-a-Nobel molecular biologist Max Ferdinand Perutz (1914-2001) and his asst. Sir John Cowdery Kendrew (1917-97) develop the first atomic model of a protein, hemoglobin, using blood from a sample of whale meat from Peru , sharing the 1962 Nobel Chem. Prize- Peru, Perutz, can do?
In 1959 chemists develop synthetic pheromones to control insect pests.

In 1960 Am. chemist Robert Burns Woodward (1917-79) (the model for nerds of the future?) announces the synthesis of chlorophyll, winning him the 1965 Nobel Chem. Prize.

In 1961 English biochemist Peter Dennis Mitchell (1920-92) pub. the Chemiosmotic Theory to explain energy storage in plants and animals via a proton gradient across a vesicular membrane that generates an electric field, countering the prevailing purely chemical phosphorylation theory and winning him the 1978 Nobel Chem. Prize after ATP synthase is discovered.



On Apr. 13, 1962 Am. chemist Richard Williams (1928-) discovers the principle behind Liquid Crystal Displays (LCDs), causing Am. engineer George Harry Heilmeier (1936-) of RCA Labs to create the first LCD in 1964.
By 1968 a total of 103 Periodic Table elements have been discovered, which are adopted as a std.


In 1968 Am. computer scientist Edward Albert Feigenbaum (1936-) and Am. molecular biologist Joshua Lederberg (1925-2008) of Stanford U. develop DENDRAL, an expert heuristic software AI system for the identification of chemical substances based on the results of spectrometric analysis.


Big year for biochemists into three-letter acronyms? In Jan. 1970 after finding natural ATP (adenosine triphosphate) to be too small for X-ray crystallography, scientists at Cambridge U. first successfully grow single giant crystals of ATP, then determine the 3-dim. structure; Creationists jump on its structure to claim that it is an example of irreducible complexity that even the simplest forms of life can't survive without; English chemist Sir John Ernest Walker (1941-) of Cambridge U. and Am. biochemist Paul Delos Boyer (1918-) later elucidate the enzymatic mechanism underlying ATP synthesis, winning them the 1997 Nobel Prize.

In 1971 Am. biochemist Bruce Nathan Ames (1928-) of UCB devises the Ames Test to determine the carcinogenicity (mutagenicity of DNA in the test organism) of chemicals by measuring the rate of mutation in Salmonella typhimurium bacteria.

In 1971 Russian-born Belgian chemist Ilya Romanovich Prigogine (1917-2003) develops Non-Equilibrium Irreversible Thermodynamics, and shows that the 2nd Law of Thermodynamics doesn't doom the Universe to a heat death, winning him the 1977 Nobel Chem. Prize.

In 1971 British pharmacologist Sir John Robert Vane (1927-2004) et al. of Wellcome Research Labs. pub. their discovery that aspirin produces its pain-relieving and fever-reducing effects by inhibiting synthesis of Prostagalandins, lipid mediators; too bad, one type of prostaglandin protects the stomach and kidney; Vane goes on to win the 1982 Nobel Med. Prize and get knighted in 1984 - in vain?



In 1972 Am. chemist Dudley Robert Herschbach (1932-), Taiwanese chemist Yuan Tseh Lee (1936-), and Hungarian-Canadian chemist John Charles Polanyi (1929-) develop a supermachine for Crossed Molecular Beam Spectroscopy to study elementary reaction processes at the molecular level, winning them the 1986 Nobel Chem. Prize.





In 1973 English environmentalist scientist James Ephraim Lovelock (1919-) of Dorset, known for proposing the Gaia Hypothesis that Earth functions as a self-regulating system proposes that chlorofluorocarbons (CFCs) have a global warming effect; in 1975 Indian atmospheric scientist Veerabhadran Ramanathan (1944-) finds that a CFC molecule is 10,000x more effective than a CO2 molecule in absorbing infrared radiation, with the soundbyte: "The effect of greenhouse gases on global warming is, in my opinion, the most important environmental issue facing the world today", causing climate scientists to eventually conclude that CFCs along with methane and other trace gases can have almost as important a climate effect as CO2. On June 28, 1974 Mexican-born Am. chemist Mario J. Molina (Jose Mario Molina-Pasquel Henriquez) (1943-2020) and Delaware, Ohio-born chemist Frank Sherwood "Sherry" Rowland (1927-2012) pub. a paper in Nature claiming that chlorofluorocarbon (CFC) propellants are accelerating the depletion of the ozone layer in the stratosphere, with one chlorine molecule able to destroy up to 100K ozone molecules, winning them the 1995 Nobel Chem. Prize; meanwhile high acidity in lakes in NE U.S. and E Canada are traced to coal-burning plants in the U.S. Midwest; on Sept. 25 Susan Solomon (1956-) and other scientists warn that continued use of aerosol sprays will cause ozone depletion, leading to an increased risk of skin cancer and global weather changes; in 1976 the Nat. Academy of Science warns against fluorocarbons in spray cans. On Sept. 13, 1976 after DuPont is first confronted with evidence that their product is destroying the stratospheric ozone layer in 1974, the U.S. Nat. Academy of Sciences reports that Freon (CFCs) used in spray cans is depleting the ozone layer of the atmosphere, increasing deadly UV radiation on the ground; in 1978 the U.S. bans CFC-based aerosols in spray cans. Watch video - Tony Heller.

In 1974 Lithuanian-born British chemist-biophysicist Sir Aaron Klug (1926-) et al. determine the crystal structure of Transfer RNA (tRNA); Klug wins the 1982 Nobel Chem. Prize, and is knighted in 1988.



In 1975 Ubiquitin, a small regulatory protein found in all eukaryotic organisms is discovered; in 1980 Israeli scientists Avram Hershko (1937-), Aaron Ciechanover (1947-), and U.S. scientist Irwin A. Rose (1926-) pub. a paper revealing the role of ubiquitin in degrading and recycling proteins, winning them the 2004 Nobel Chem. Prize.




In 1976 Kiwi chemist Alan Graham MacDiarmid (1927-2007), Am. physicist Alan Jay Heeger (1936-), and Japanese chemist Hideki Shirakawa (1936-) of the U. of Penn. discover that bromine doping greatly increases the electrical conductivity of polyacetylene, followed next year by iodine, winning them the 2000 Nobel Chem. Prize for Conductive Polymers; in 1978 MacDiarmid, L.E. Lyons, and Sir Nevill Francis Mott (1905-96) create Organic Semiconductors, consisting of carbon compounds doped with oxygen.


In 1977 a team led by English molecular biologist Frederick Sanger (1918-2013) and his student Allan Maxam (1942-) of Harvard U. determine the exact DNA sequence of bacteriophage phi-X174, the first for any organism, using the Maxam-Gilbert Sequencing Method that combines chemicals that cut DNA at specific bases with radioactive labeling and polyacrylamide gel electrophoresis, becoming the first DNA sequencing, becoming a breakthrough in molecular biology; Am. biochemist Walter Gilbert (1932-) independently develops a nucleotide sequencing technique, and Sanger and Gilbert share the 1980 Nobel Chem. Prize.

In 1982 Am. chemist Thomas Robert Cech (1947-) discovers that RNA can act as a catalyst and participate in cellular reactions, incl. on ribosomes; in 1984 Sidney Altman (1939-) independently discovers Catalytic RNA, winning them the 1989 Nobel Chem. Prize.



In 1985 English chemist Sir Harold Walter "Harry" Kroto (1939-) of the U. of Sussex, and Am. chemists Robert Floyd Curl Jr. (1933-), Richard Errett Smalley (1943-2005) et al. of Rice U. discover Buckminsterfullerenes (Buckyballs) (Carbon 60), for which they receive the 1996 Nobel Chem. Prize; Japanese physicist Sumio Iijima (1939-) of Ariz. State U. develops a tube-shaped variety, Carbon Nanotubes; one of the spinoffs is Buckypaper, which is 10x lighter and 500x stronger than steel.

On Mar. 23, 1989 Univ. of Utah chemists Bobby Stanley Pons (1943-) and Martin Fleischmann (1927-2012) announce that they have achieved cold fusion; too bad, other researchers fail to verify it, and it is eventually labelled an "illusion" by Time mag. - too much cold brewsky in Mormonland?

In 2003 Am. chemist Carolyn Ruth Bertozzi (1966-) coins the term Bioorthogonal Chemistry to refer to any chemical reaction that can occur inside living systems without interfering with native biochemical processes.

In 2008 Japanese organic chemist Osamu Shimomura (1928-2018) isolates glowing green fluorescent protein (GFP) in the jellyfish, winning him a share of the 2008 Nobel Chem. Prize.
On Apr. 30, 2010 Cell announces that researchers at UCLA led by Hong Zhou have used a new type of cryo-electron (cryo-EM) microscope to see atoms for the first time in a virus with 3.3 angstrom resolution.
In Aug. 26, 2010 Angewandte Chemie pub. work by MIT prof. Christopher Cummings et al. that instead of using chlorine, phosophorus can be attached to organic compounds using UV light.
On Nov. 24, 2010 scientists at the U. of Nottingham announce a breakthrough allowing them to build 3-D nanomolecular structures on a 2-D surface.
On Feb. 10, 2011 Angewandte Chemie pub. the discovery by researchers at Va. Commonwealth U., McNeese State U., Peking U., and John Hopkins U. of Superhalogens.
On Apr. 3, 2011 researchers at Penn. State U. announce a new sixth type of symmetry in materials, rotation reversal; the other five are rotation, inversion, rotation inversion, translation, and time reversal.
On June 17, 2011 Science pub. the creation of the first crystals from metallic glass by Wendy Mao et al. of the U.S. Dept. of Energy.
On June 5, 2013 chemists at the U. of Pittsburgh pub. their development of titanium dioxide on a stick of carbon nanotubes which might be used to create a sensor to measure blood sugar from human breath samples.
On June 20, 2013 Niek van Hulst et al. of the U. of Glasgow and the ICFO-Inst. of Photonic Sciences pub. an article in Science reporting the first direct observation of the quantum effects in photosynthesis, revealing that coherence maintains high levels of transport efficiency and adapts to environmental influences.
In June 2013 Richard Crooks of UTA and Ulrich Tallarek of the U. of Marburg pub. an article in Angewandte Chemie describing their new technique of electrochemically-mediated seawater desalination, which uses a small electrical field to desalinate seawater sans membranes.
On Oct. 28, 2013 Jerry Qi et al. of the U. of Colo. Boulder announce the incorporation of "shape memory" polymer fibers into the composite materials used in traditional 3-D printing to create a form of 4-D printing where an object fixed in one shape can later be changed to take on a new shape.
On Dec. 13, 2013 P. Michael Conn of the Oregon Primate Research Center et al. announce the discovery of pharmacoperones, which fix misfolded proteins.
On Feb. 7, 2014 Ali Tavassoli et al. of the U. of Southampton pub. an article in Angewandte Chemie International Edition announcing the first workable Click Chemistry, the artificial joining of oligonucleotides.
On July 17, 2015 researchers at Eindhoven U. of Technology and FOM Foundation pub. an article in Nature Communications announcing the new material gallium phosphide, which can enable a solar cell to produce hydrogen gas from liquid water and sunlight.
On Nov. 6, 2017 Ramanarayanan Krishnamurthy et al. of Scripps Research Inst. pub. an article in Nature Chemistry claiming the discovery of synthetic enzyme diamidophosphate (DAP), which drives phosphorylation, claiming it might be the missing link to the first life on Earth.
On Dec. 28, 2020 researchers at Scripps Research pub. a study in Angewandte Chemie describing their discovery that the simple compound diamidophosphate (DAP) could have chemically combined to create DNA building blocks called deoxynucleosides into strands of primordial DNA.